HISTORY OF
ARTHROSAMID®
Arthrosamid® (a registered trademark of Contura A/S.).
Arthrosamid’s story started two decades ago. The brainchild of the team at Contura, the product had been in human use for various indications since 2000, and a favourable safety profile had already been established.

ARTHROSAMID® — AN OA TREATMENT 20 YEARS IN THE MAKING
In April 2021, Arthrosamid received CE mark (European market approval) for the symptomatic treatment of patients with knee osteoarthritis (OA), following the completion of a twelve-month prospective open label study which saw participants experience significant pain reduction1,
The CE mark for Arthrosamid represented a major milestone for a product that has been in development for more than 20 years — and fulfils an unmet clinical need for an effective, long-acting, safe and minimally invasive treatment that may postpone and potentially prevent knee surgery for those with OA.2 But what’s the backstory behind this novel, non-biodegradable hydrogel that is now expected to provide OA patients with life changing pain relief and improved mobility? 1,3,4
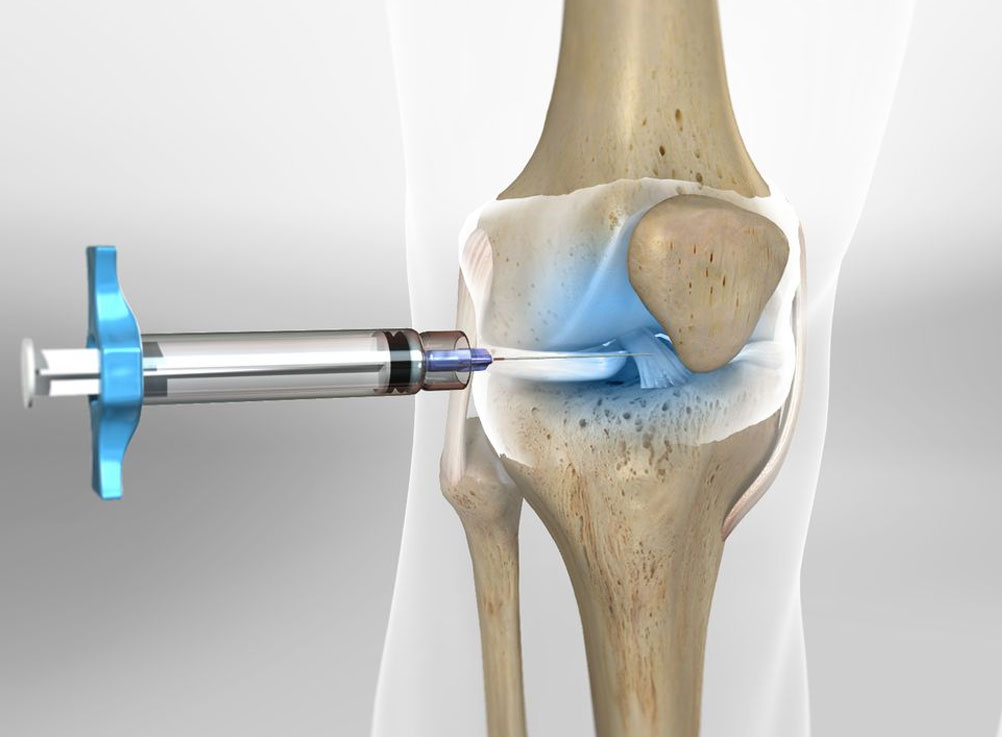
How does it work?
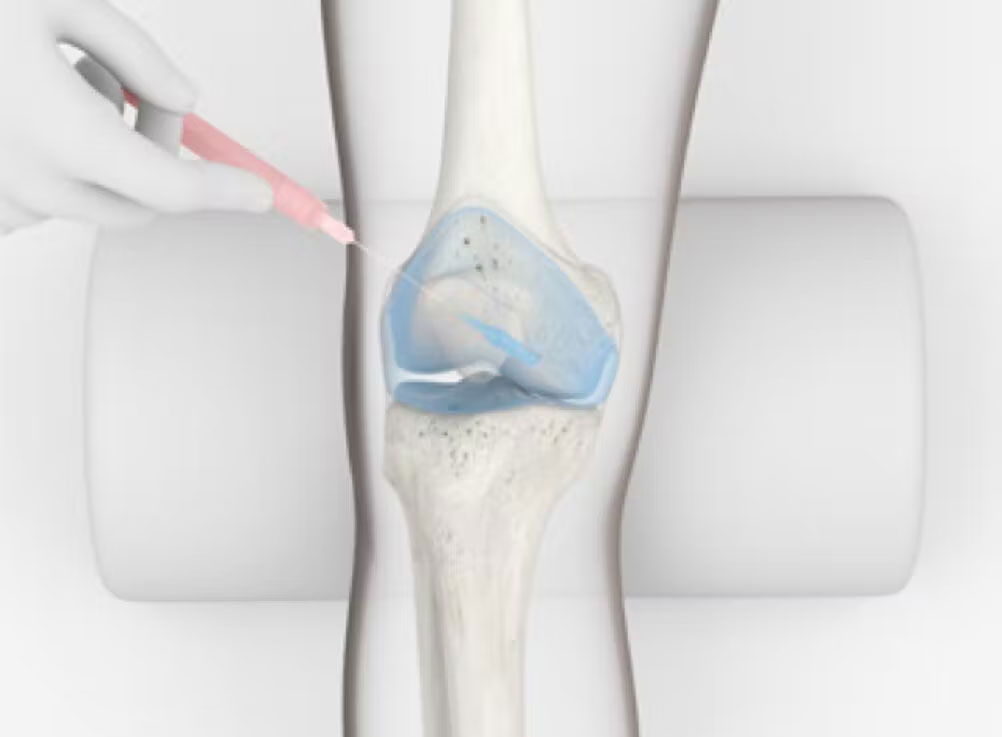
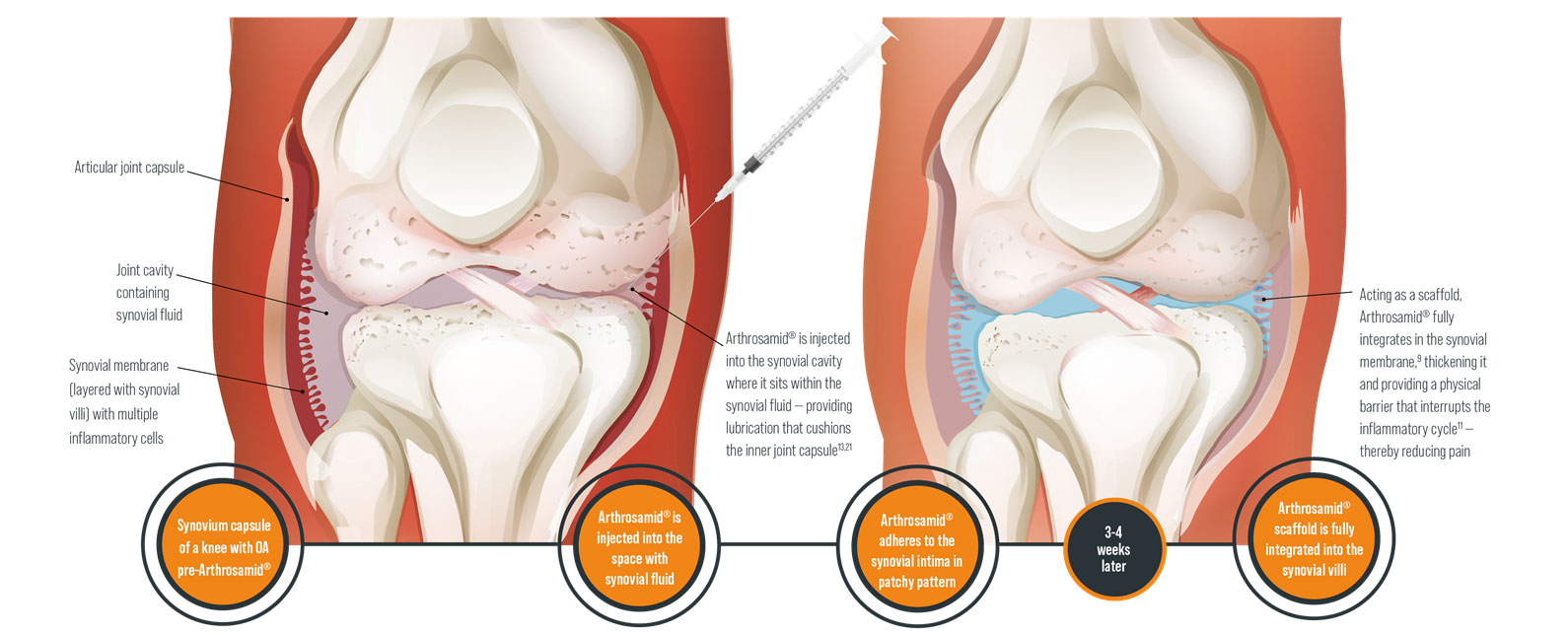
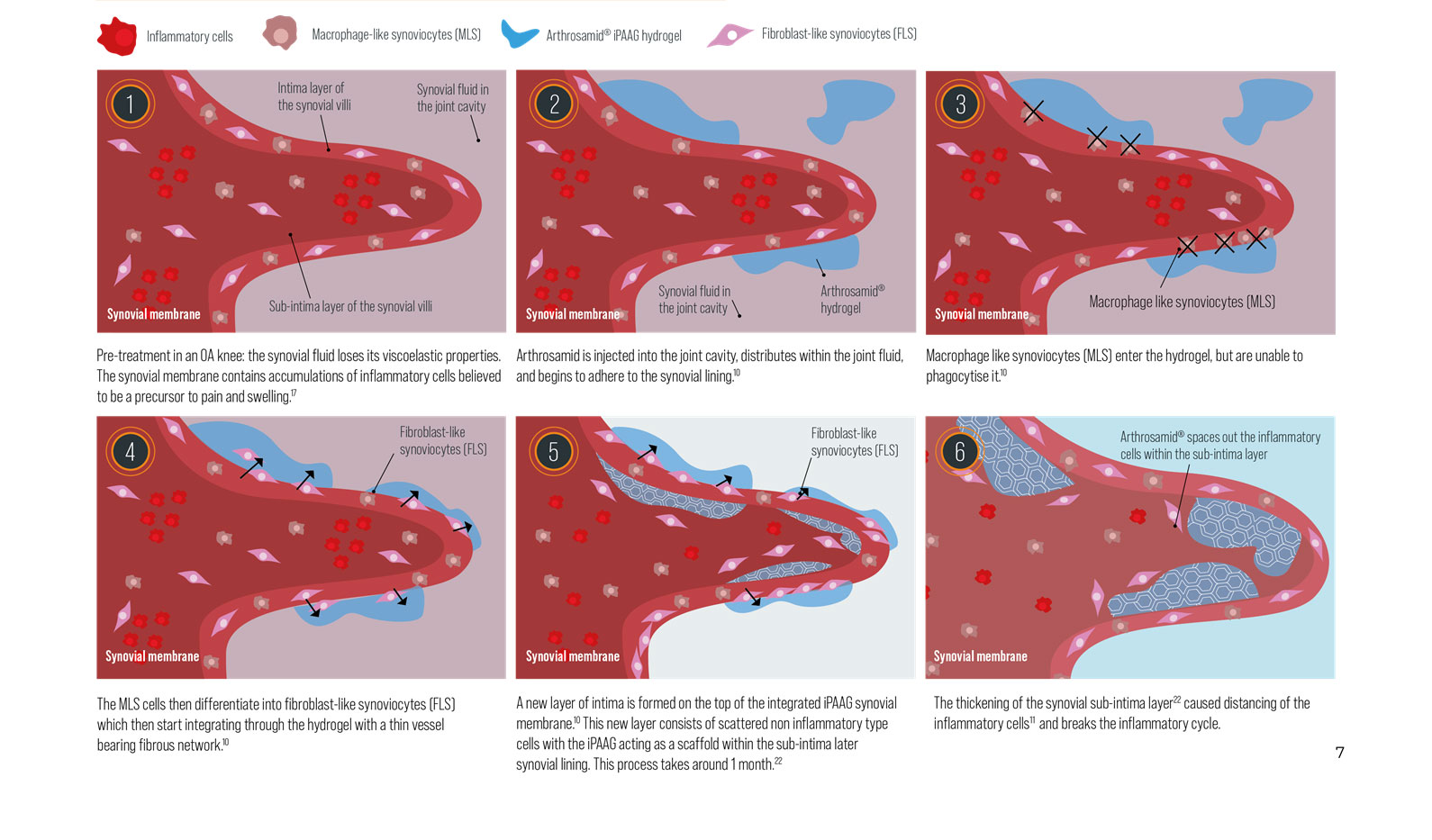
Arthrosamid® is an intra-articular polycrylamide hydrogel injection for the symptomatic treatment of knee osteoarthritis. Once injected into the inner joint cavity, it restores viscosity within the synovial fluid, improving lubrication and cushioning of the joint. Arthrosamid also integrates into the synovium of the inner joint capsule creating a cushion-like effect.
What are the benefits?
As Arthrosamid works to cushion the joint, it can reduce your pain, decrease stiffness and help movement. The hydrogel itself does not degrade and therefore it provides long-acting relief, improving your quality of life. In clinical trials, patients reported a significant reduction in their pain symptoms by week 4 after their injection and unlike other injectable treatments, the level of reduction was maintained at the 1 year follow up period.
What is Arthrosamid®?
Arthrosamid is a new type of treatment for knee osteoarthritis that offers patients an effective alternative to the currently available therapies. Based on an innovative non-biodegradable hydrogel technology, Arthrosamid is 97.5% water with 2.5% being a cross linked polyacrylamide backbone. When injected into the knee Arthrosamid cushions the joint and reduces pain, providing safe and sustained relief, all with one injection.
When can I go back to normal activity?
You will have to avoid any strenuous weight-bearing activities (e.g. running, tennis or long walks) during the first few days after your injection. We will advise you on how to slowly introduce more activity.
PRODUCT OF
ARTHROSAMID®
Arthrosamid’s story started two decades ago. The brainchild of the team at Contura, the product had been in human use for various indications since 2000, and a favourable safety profile had already been established.
ARTHROSAMID®
In April 2021, Arthrosamid® received CE mark (European market approval) for the symptomatic treatment of patients with knee osteoarthritis (OA), following the completion of a twelve-month prospective open label study which saw participants experience significant pain reduction1,
The CE mark for Arthrosamid® represented a major milestone for a product that has been in development for more than 20 years — and fulfils an unmet clinical need for an effective, long-acting, safe and minimally invasive treatment that may postpone and potentially prevent knee surgery for those with OA.2 But what’s the backstory behind this novel, non-biodegradable hydrogel that is now expected to provide OA patients with life changing pain relief and improved mobility? 1,3,4
What are the side effects?
In a number of clinical trails with Arthrosamid®, there were no serious side effects related to hydrogel. The most commonly reported side effects were mid to moderate injection site pain and swelling, witch were generally transient in nature. The overall safety profile of the hydrogel has been established over the last 20 years with its use for various indications in the body.
How does Arthrosamid® work?
Arthrosamid® is the only approved medical device that integrates into the synovial tissue of the inner capsule and decreases joint stiffness, thereby diminishing pain and improving function of the knee affected by QA. Its permanence means that it can provide long-term pain relief to treat knee QA.

Mode of Action - let's take a closer look
Subscribe to our mailing list
Recent Post
-
Decoding the Choice: A Step-by-Step Patient Guide to Selecting the Best Knee Gel Injection for Osteoarthritis Relief03 Dec 2025
-
Innovative Ways to Manage Patellofemoral Pain Syndrome: From Exercise Selection to Everyday Movement Strategies01 Dec 2025
-
The Science Behind Knee Gel Injections: How Synovial Fluid Supplements Relieve Osteoarthritis Pain01 Dec 2025