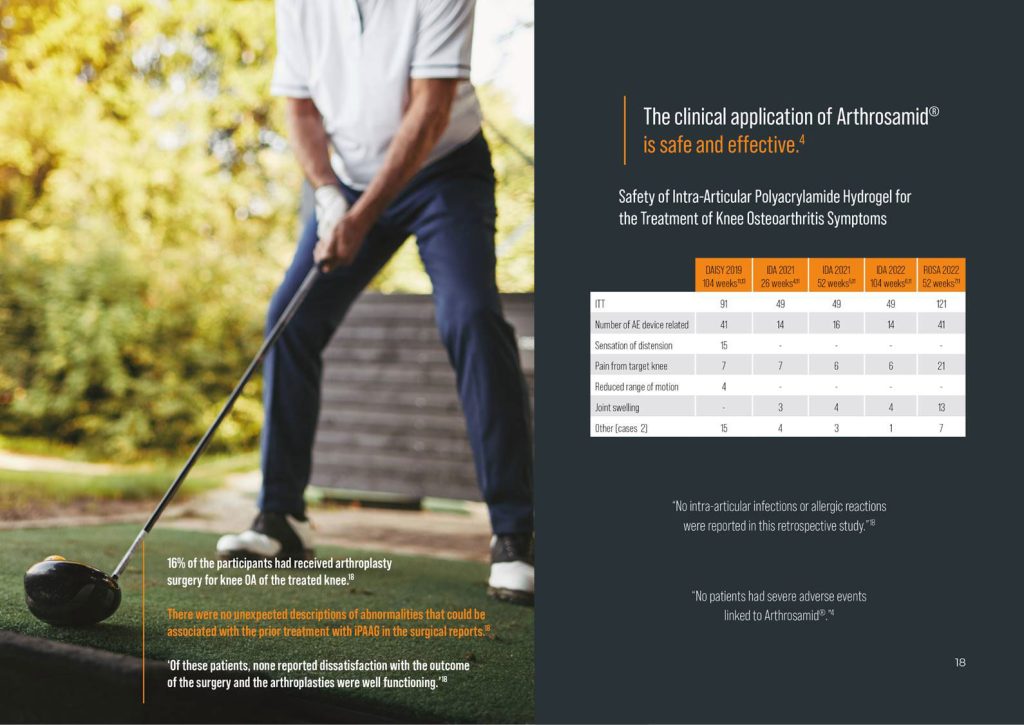
The clinical application of Arthrosamid is safe and effective.1,2

Cole A., Maulana R., Whitehead J.P., Lee P.Y.F., A Systematic Review of the Novel Compound Arthrosamid Polyacrylamide (PAAG) Hydrogel for Treatment of Knee Osteoarthritis. Medical Research Archives, European Society of Medicine. Vol 10 No 8 (2022): VOl.10 Issue 8, ISSN: 2375-1924. (Link to download paper)

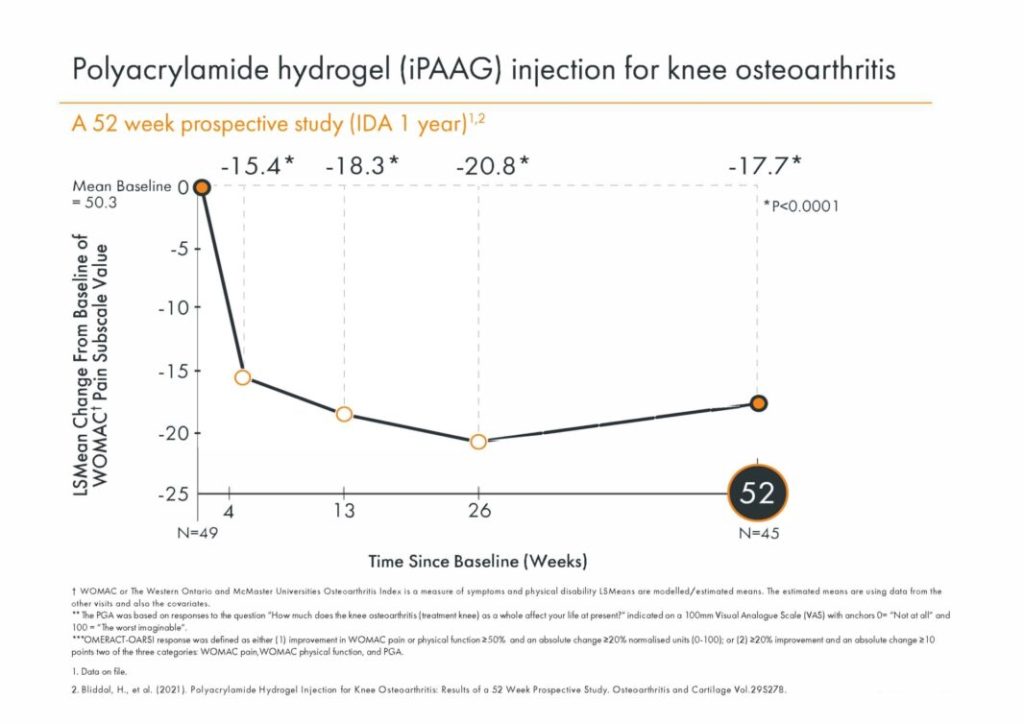
Bliddal, H., et al. (2021). Polyacrylamide Hydrogel Injection for Knee Osteoarthritis: A 6 Months Prospective Study. J Orthop Res Ther. 6(2). 1188. ISSN 2575-8241.
Statistically significant reduction in painmaintained at
2 years

Bliddal, H., et al. (2022). A Prospective Study of Polyacrylamide Hydrogel Injection forKnee Osteoarthritis: Results From 2 Years After Treatment. Poster presented at OARSI 2022.Osteoarthritis and Cartilage Vol.30, Supplement 1, S371-S372. DOI: 10.1016/j.joca.2022.02.499
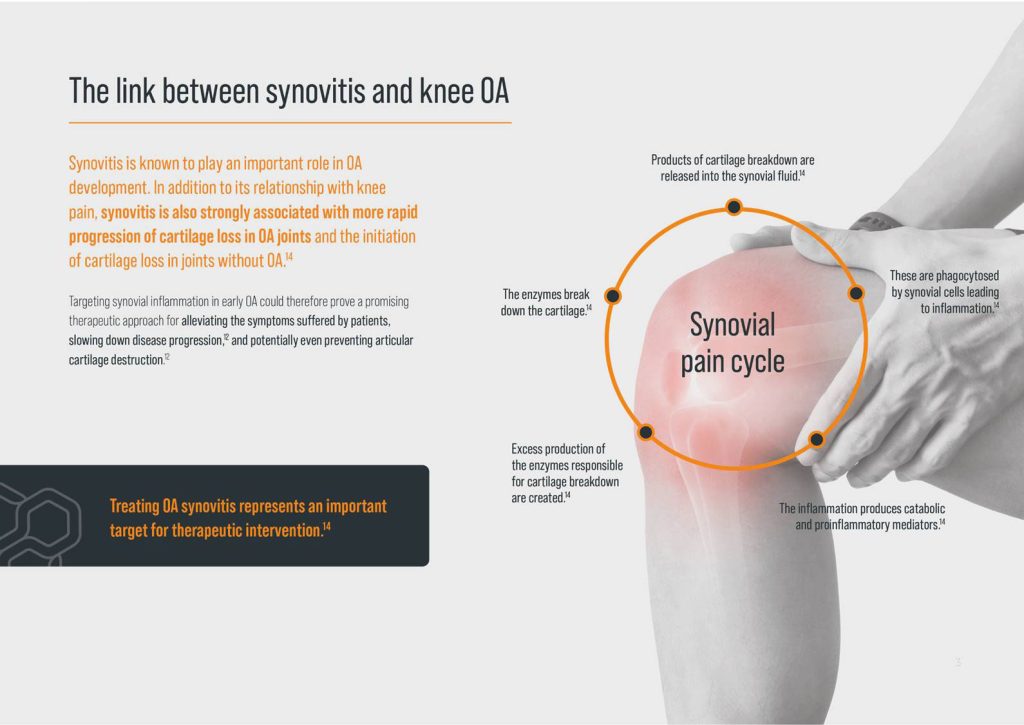
Significantly Reduction in Patellofemoral Bone Marrow Lesions at 6 months

Maulana R, Cole A, Lee P.Y.F. Reduction in Patellofemoral Bone Marrow Lesions Following Single Arthrosamid Intra -Articular Injection of Polyacrylamide Hydrogel (ipaag) in the Treatment of Advanced Osteoarthritis. J Arthritis, 2022, 11(3), 024-026 (Link to download paper)
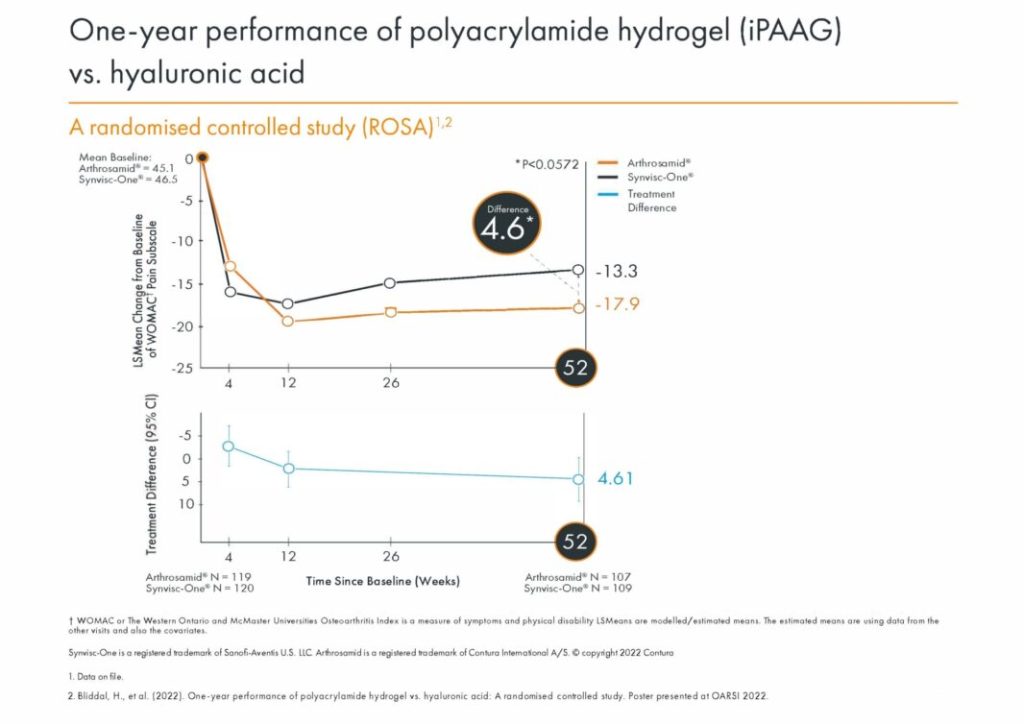
Significant difference in change from baseline between Arthrosamid and Synvisc-One at 12 months.

Bliddal, H., et al. (2022). One-year performance of polyacrylamide hydrogel vs. hyaluronic acid in age, BMI, and Kellgren-Lawrence subgroups: A subgroup analysis of a randomised study. Poster presented at OARSI 2022. Osteoarthritis and Cartilage Vol.30, Supplement 1, S373-S374.